Despite advantages to its use, the procedure remains underutilized. Two experts outline an approach for getting started.
Hysteroscopy is a technique through which the uterine cavity can be evaluated visually to assess for structural abnormalities. The first hysteroscopy was performed by Pantaleoni in 1869, and since then emerging technologies have greatly advanced the scope of this technique. 1
Improvements in hysteroscopes, optics, distension media, anesthetics, and hysteroscopic instruments have made office hysteroscopy (OH) more feasible and less painful for patients. 2 Advantages to OH include avoidance of general anesthesia, patient and physician convenience, faster recovery, and cost-effectiveness. 3
The costs of OH vs hysteroscopy in the operating room (OR) have been reviewed for various indications. OH has been found to be cost-effective for evaluation of abnormal uterine bleeding (AUB), infertility, and triage of intracavitary pathology, ranging from negative to complex pathology. 4-6 In one study assessing cost-effectiveness of OH over OR hysteroscopy to evaluate AUB, the cost savings was found to be $1498 per patient. 4 OH has also been found to be safe and effective, with a complication rate of 0% to 1.5%. 4-6 Study findings have revealed high success rates for diagnostic OH, with rates as high as 94.8%. 7
Not only has OH been shown to be effective, but it has also been found to have high patient satisfaction regarding comfort and convenience. 8,9
In one study of patients undergoing OH vs hysteroscopic polypectomy in the OR, 95% of women who underwent OH stated they would prefer OH again. Women were also found to have decreased postoperative analgesic requirements, decreased time away from home, and decreased recovery time. 8
Despite these advantages, OH remains underutilized. In a study by Shields et al, among 305 hysteroscopies, only about 25% were performed in the office. 6 This discrepancy may be due to limited physician training, limited availability in clinics, concerns with start-up costs, and fears of patient discomfort.
This article will review current best practices for OH and provide strategies for practicing ob-gyn specialists who hope to incorporate OH into their practice.
Hysteroscopy has a wide range of diagnostic and therapeutic uses. Some of the more common uses include diagnosis and management of abnormal uterine bleeding (AUB) and postmenopausal bleeding, including polyps, submucosal fibroids, and thickened endometrium. Importantly, OH can be used to rule out structural abnormalities to prevent unnecessary trips to the operating room.
Table. Potential uses of hysteroscopy

AUB accounts for 30% of all gynecologic visits to the specialist obstetrician-gynecologist. 10 Diagnosis has long relied on a combination of tissue sampling and ultrasound despite their low diagnostic accuracy for intracavitary pathology. Although transvaginal ultrasound is helpful for evaluating the myometrium, its sensitivity and specificity for evaluating intracavitary pathology are only 56% and 73%, respectively. 11
A systematic review showed that endometrial biopsy has high accuracy when assessing for a global process, such as endometrial cancer or hyperplasia, but if cancer occupies less than 50% of the endometrial cavity surface, it is likely to be missed.12 In a meta-analysis that evaluated the accuracy of diagnostic hysteroscopy compared with guided biopsy during hysteroscopy, operative hysteroscopy, or hysterectomy, diagnostic hysteroscopy had an overall success rate of 96.6%, and abnormalities were found in 46.6% of premenopausal and postmenopausal women with AUB. 13
Hysteroscopy can be also used in the work-up of infertility and can aid in the diagnosis and treatment of uterine septa and Asherman syndrome. Additional uses include removal of retained intrauterine devices, targeted biopsy, diagnosis of isthmoceles, and removal of retained products of contraception.
Hysteroscopes come in a variety of sizes, lens angles, and capabilities. When starting an office hysteroscopy program, consider what procedures will be performed. There are diagnostic hysteroscopes, which may offer a smaller outer diameter but lack the attachments for anything but inflow, and there are operative hysteroscopes that have a larger outer sheath but allow for the passage of instruments including scissors, graspers, tenacula, and biopsy forceps.
Consider the size of instruments used in other common gynecologic procedures. For example, a typical endometrial biopsy pipelle is 3 mm, whereas an intrauterine device insertion sheath can be 4 to 5 mm. Diagnostic and operative hysteroscopes come in sizes as small as 2.8 and 4 mm, respectively, so these sizes may offer the physician and patient further comfort.
Lenses come in different angles as well. Typically, a 30° lens goes well with a diagnostic hysteroscope, as it provides the ability to see around acute angles with little movement. A 12° lens is more compatible with an operative hysteroscope since the operative equipment can be better seen directly in front of the lens.
Lastly, some may consider a rigid, flexible, or disposable hysteroscopy system. Rigid hysteroscopes allow for vaginoscopy and more robust operative procedures to be performed. They often require a large capital investment but can be more cost-effective and less wasteful than disposable systems when used frequently.
Flexible hysteroscopes provide the user the ability to see around acute angles without touching sensitive cervical walls.
However, flexible hysteroscopes typically have fiber-optic cables, which are expensive to maintain, and the systems are often limited to diagnostic capabilities only. Flexible scopes are often not able to perform vaginoscopy, which is the recommended technique for pain control.
Disposable hysteroscopes offer an alternative to the high up-front costs and sterilization needs of reusable hysteroscopes. However, the per-use cost can be prohibitively high, and the plastic medical waste created is an environmental problem. Most of these have a 3- to 4-mm sheath with limited ability to perform operative procedures.
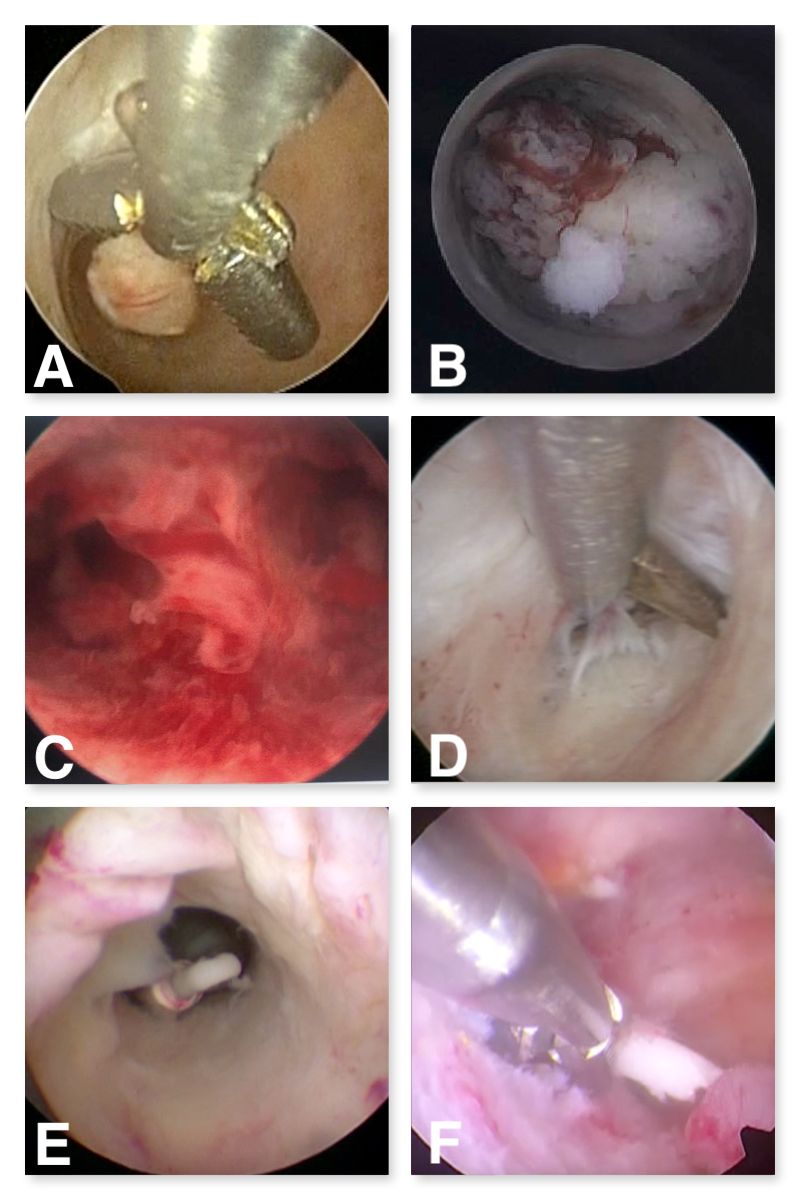
When performing operative hysteroscopy, consideration must be given to the type of tools needed. Most basic office procedures can be done with scissors (polypectomy, lysis of adhesions, metroplasty) and grasper (directed biopsy, intrauterine device (IUD) removal, products of conception removal).
The authors prefer the addition of a hysteroscopic tenaculum to allow for intact removal of large polyps by providing a more robust grip than a grasper. Although biopsy forceps can be useful for directed biopsies, a grasper can provide the same functionality while also being able to grasp retained IUDs.
Mechanical tissue extraction devices are also available, whether operated manually by hand or powered. Some of these are paired with a tabletop suction device or fluid management system. There are electrosurgical instruments available for the office as well, but the authors prefer to avoid electrosurgery in the office to reduce the risk of complications, such as injury to surrounding structures and perforation.
However, the 15 French bipolar resectoscope and 5 French bipolar twizzle can be used with a generator if the physician prefers energy for procedures like metroplasty or myomectomy.
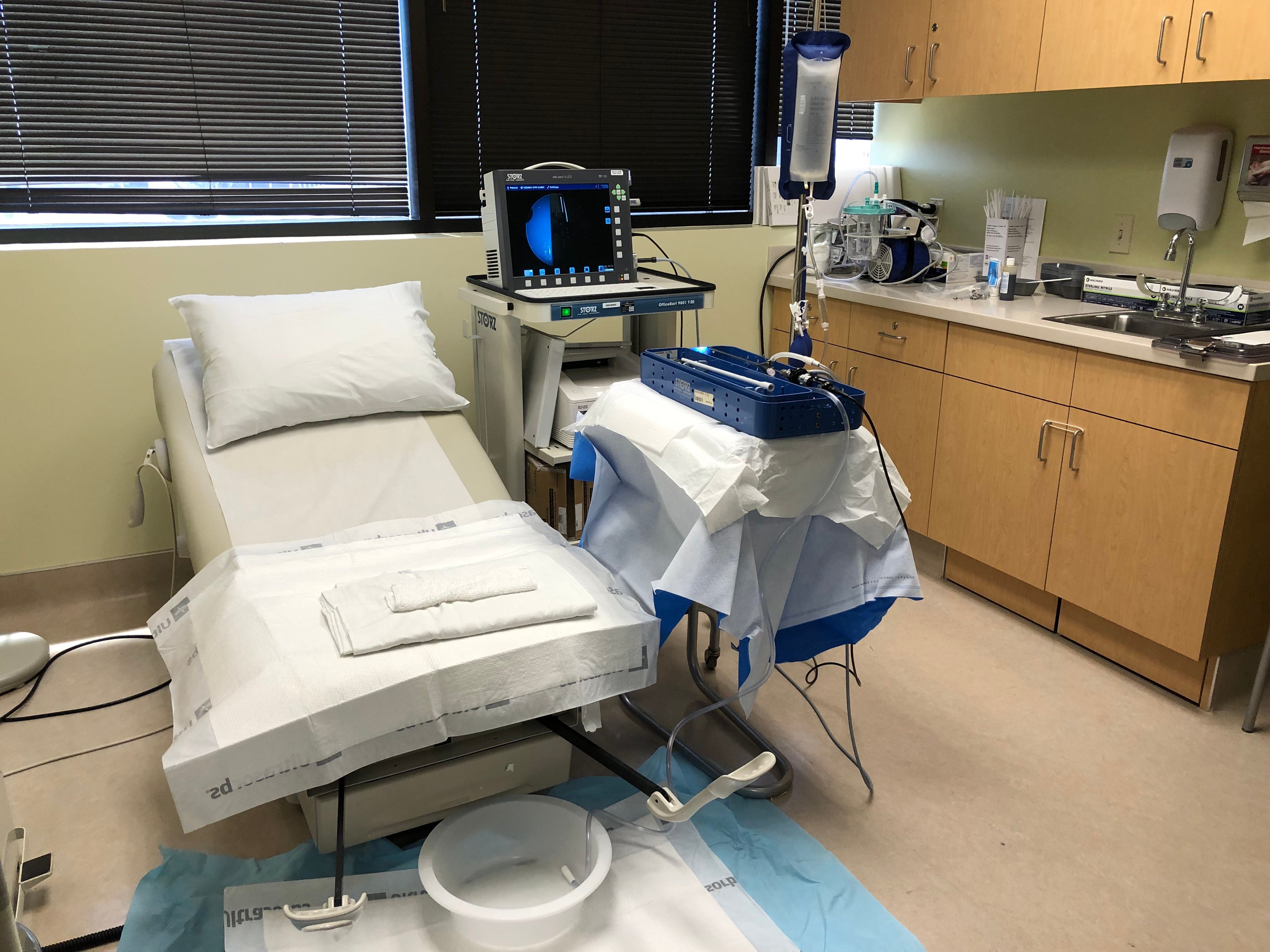
Hysteroscopy can be done in a small area like a converted exam room (Figure 1). An exam table that rises and lowers is preferred. In addition to the hysteroscope, a visualization system is required, such as a camera, light source, and monitor, as well as the ability to capture images for the electronic health record (Figure 2).
Inflow and outflow tubing, operative port seals, and a way to measure inflow and outflow are necessary. The authors prefer using a 1-L normal saline bag in a pressure bag for inflow, with the outflow hung to gravity into a basin or under-buttocks drape. A mayo stand or cart can be helpful for setting the equipment on. In addition, a speculum, tenaculum, dilators, and local anesthesia should be available for use if needed. Most importantly, a cleaning process is required for all reusable instruments including high-level disinfection or autoclaving in office or through a centralized sterile processing unit.
The most widely used distension medium in the office setting is normal saline because it is ubiquitously available and comes in multiple bag sizes. Most office hysteroscopy settings will not use a fluid management device, as these are large and have expensive tubing, canister costs, and waste requirements. The physician will need a way to manually calculate the fluid deficit.
This can be performed by measuring outflow in a graded under-buttocks drape or a basin at the foot of the bed. If the hysteroscope has no outflow channel, typically the amount of inflow is so small that a true deficit does not need to be calculated. The recommended deficit limit for normal saline in healthy patients is 2500 mL; however, in elderly patients or those with comorbidities, a lower threshold may be considered.14 If the physician anticipates doing procedures in the office that could go above this limit, a fluid management device should be utilized.
Appropriate patient selection is the most important component for both patient and physician comfort when starting an office hysteroscopy program.
A prospective cohort study showed that nulliparity, cervical pathology, and procedures over 2 minutes in length were correlated with severe pain.15 Physicians may want to start with diagnostic hysteroscopy in multiparous patients without cervical pathology to allow for building confidence to progress to operative hysteroscopy.
Anesthesia and analgesia have been well studied for office hysteroscopy. A 2020 systematic review found that nonsteroidal anti-inflammatory drugs (NSAIDs) significantly reduced observed and reported pain during and after office hysteroscopy. Opioids and antispasmodics reduced observed pain during the procedure but were associated with a higher risk of adverse effects (AEs).
Therefore, women without contraindications should be advised to take oral NSAIDs prior to office hysteroscopy. 16 Another 2020 meta-analysis of over 1300 patients showed that when data were aggregated, all anesthetics had some benefit, including topical, transcervical, intracervical, paracervical, and intracornual sites. 17
However, every type of anesthesia included studies that favored placebo, and several randomized, controlled trials have reported increased pain, bradycardia, and hypotension after cervical injection. 18,19 Local anesthesia did not reduce the risk of procedural failure.
The authors choose to use vaginoscopy as the main approach for reducing pain. Vaginoscopy involves using no speculum or tenaculum and placing the camera directly into the patient’s vagina to allow for distention with saline.
Rotation of the light cord then allows for guidance of the angled hysteroscope along the cervical canal and into the uterus. Hydrodistension of the canal promotes passage even with difficult angles or stenosis. Large studies enrolling thousands of patients over 25 years of age have found that a vaginoscopic approach has decreased pain compared with traditional hysteroscopy with a speculum with no difference in procedural success rates. 20-22
The use of hysteroscopy for the diagnosis and treatment of intrauterine pathology: ACOG Committee opinion, number 800. Obstet Gynecol. 2020;135(3):e138-e148. doi:10.1097/AOG.0000000000003712
AAGL Advancing Minimally Invasive Gynecology Worldwide, Munro MG, Storz K, et al. AAGL practice report: practice guidelines for the management of hysteroscopic distending media: (replaces hysteroscopic fluid monitoring guidelines. 2000;7:167-168). J Minim Invasive Gynecol. 2013;20(2):137-148. doi:10.1016/j.jmig.2012.12.002
Physician fee schedule search. Centers for Medicare & Medicaid Services. https://www.cms.gov/medicare/physician-fee-schedule/search
The authors also do not routinely use medical cervical dilation for pain control. A meta-analysis evaluating use of cervical preparations to reduce pain during outpatient hysteroscopy showed no reduction in pain in premenopausal or postmenopausal women who required cervical dilation of 5 mm or less, which is the maximum size of our operative hysteroscope. 23 Preprocedural misoprostol can be associated with AEs such as cramping, fever, and diarrhea, making the patient uncomfortable before the procedure even starts.
Lastly, efforts to reduce preprocedural anxiety should be employed. Another meta-analysis found that pain experienced during hysteroscopy is worsened by preprocedural anxiety. Wait time before the procedure can increase anxiety, and music during the procedure may reduce anxiety. 24
It is important to counsel patients on expectations and let them know that the procedure can be stopped at any time. Patients who prefer a procedure under sedation should be accommodated in an operating room or surgery center.
All patients should be involved in a fully informed consent process and booked when an optimal view of the endometrial cavity is achievable. Patients typically are booked immediately after cessation of menses or optimally cycle days 4 through 11.
Considerations for starting OH in the clinic include startup cost, reimbursement, coding, and setting up appropriate procedures for sterilization.
In 2017, relative value units (RVUs) for office operative hysteroscopy (CPT code 58558) went up by 237%, whereas RVUs for OR hysteroscopy cases went down by 11%.25 These changes reflect that payers recognize the benefits of OH and are committed to facilitating and incentivizing its use. Estimates place equipment start-up costs between $15,000 and $35,000 based on whether the equipment is new or used, the number of operative trays, and the style of camera and monitor system. 26,27 With the increased reimbursement, physicians and practices have the potential to break even with less than 50 office operative hysteroscopies.
Multiple models have been devised for hysteroscopy simulation, however, there are limited studies on clinical utility and outcomes. 28 Models include animal organs, vegetables, synthetic uteri, and virtual reality.
The American College of Obstetricians and Gynecologists (ACOG) has devised a module for hysteroscopy simulation for resident education that uses a synthetic uterine model and a vegetable model to increase skills and knowledge, including hysteroscope assembly as well as the ability to maneuver the hysteroscope and operative tools. 29 Virtual models, including the HystSim (VirtaMed), have become more realistic and allow for a range of procedures, but require further validation before inclusion in standardized courses.
Hysteroscopy can be performed safely in the office and has high efficacy and patient satisfaction; however, there are still low rates of OH in the United States. Additionally, OH has been found to be cost-effective, and physician reimbursement rates have increased for OH compared with hysteroscopy in the OR. Setup should be relatively simple and requires communication with clinic staff to ensure that the patient is comfortable and that equipment can be available and sterilized.
Considering the benefits to physicians and patients, OH should be considered a first-line option for the diagnosis and treatment of a variety of uterine pathology.
OH can also serve to triage cases, preventing negative cases from going to the OR and ensuring that more complex pathologies can be done in the appropriate setting.
References